Sciwind Biosciences Announces Publication of Phase III SLIMMER Trial Results in The Lancet Diabetes & Endocrinology and Oral Presentation at ADA Scientific Sessions
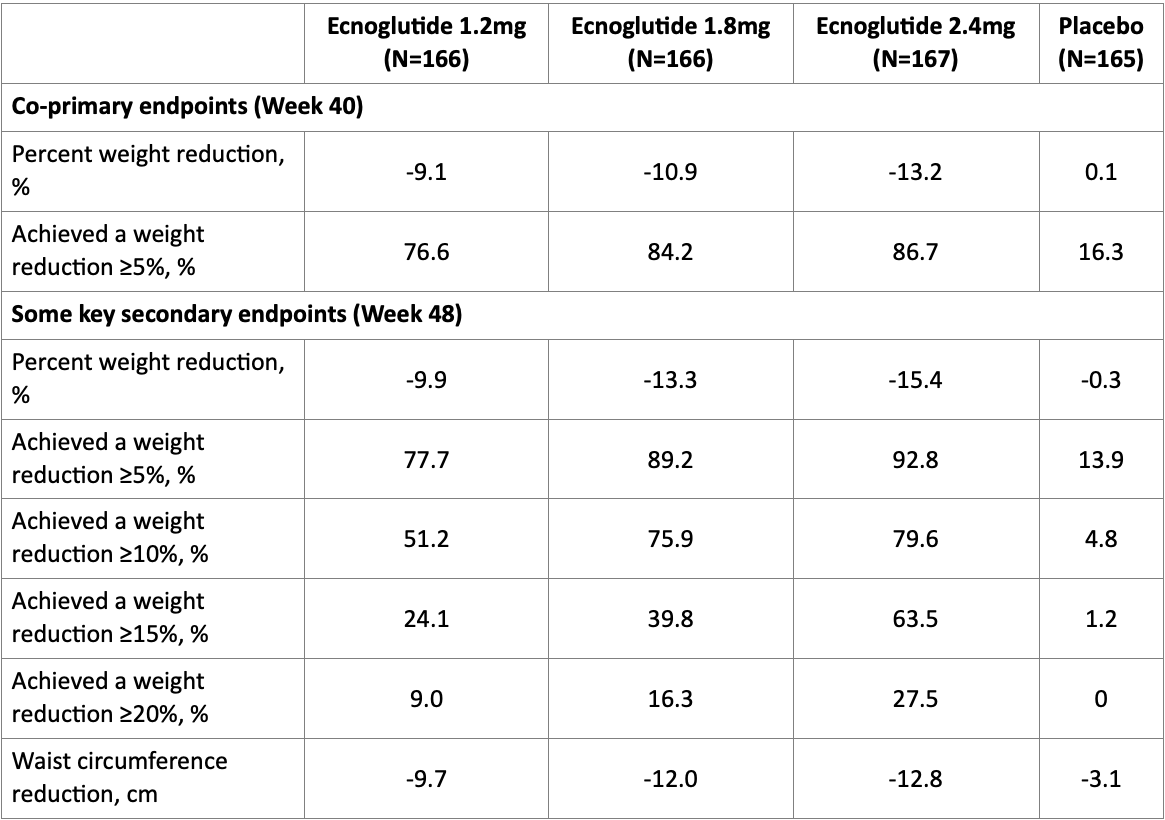
Hangzhou, China & San Francisco, U.S. – June 21, 2025 – Sciwind Biosciences, a clinical-stage biopharmaceutical company focused on metabolic diseases, today announced Phase III SLIMMER trial results evaluating ecnoglutide (XW003) in Chinese adults with overweight or obesity. The findings, published in The Lancet Diabetes & Endocrinology and presented orally at the 85th American Diabetes Association (ADA) Scientific Sessions, show that ecnoglutide, the first cAMP-biased GLP-1 receptor agonist, achieved a 15.4% mean weight loss, with 92.8% of participants achieving ≥5% weight loss at week 48.
Professor Linong Ji from Peking University People's Hospital is both the first author and the corresponding author of this paper. In addition, Qing Zheng from Sciwind Biosciences serves as co-corresponding author.
Ecnoglutide represents a significant scientific advancement as the first clinical validation of Nobel Prize-winning GPCR biased agonism theory in metabolic disease. Unlike unbiased GLP-1 therapies, ecnoglutide selectively activates cAMP signaling pathways while minimizing β-arrestin recruitment, a mechanism that may explain its enhanced efficacy and sustained metabolic effects.
The SLIMMER trial was a multicenter, randomized, double-blind, placebo-controlled study conducted across 36 sites in China. A total of 664 adults with BMI ≥28 kg/m² or BMI ≥24 kg/m² plus at least one weight-related comorbidity were randomized to receive once-weekly subcutaneous ecnoglutide (1.2 mg, 1.8 mg or 2.4 mg) or placebo for 48 weeks. The coprimary endpoints were the percentage change in body weight from baseline and a weight reduction of ≥5% at week 40.
Using the treatment policy estimand, at week 40, ecnoglutide demonstrated dose-dependent weight loss, with the highest dose (2.4 mg) achieving a mean 13.2% reduction from baseline versus a 0.1% increase for placebo (p<0.0001). By week 48, weight loss further improved to 15.4% (placebo-adjusted 15.1%), with 92.8% of participants achieving clinically meaningful ≥5% weight loss - nearly seven times the placebo response rate. Notably, 79.6% and 63.5% of participants achieved ≥10% and ≥15% weight reduction, respectively, numerically exceeding results seen with other unbiased GLP-1 therapies in similar populations.
The primary and key secondary endpoints of the study showed statistically significant superiority to placebo with p-values < 0.0001.
The treatment policy estimand assesses efficacy irrespective of treatment adherence or concomitant use of glucose-lowering/weight-affecting therapies.
Accompanied by clinically meaningful weight loss, ecnoglutide treatment significantly improved other key cardiometabolic risk factors (including waist circumference, blood pressure, lipid profile, HbA1c, fasting glucose, insulin level, and HOMA-IR) along with a notable reduction in uric acid levels, up to 54.3 µmol/L, and lower hyperuricaemia incidence.
In addition, ecnoglutide significantly reduced liver fat content. Among participants with a baseline liver fat content ≥ 8%, the mean percentage change from baseline in liver fat content at week 40 reached -53.1% in the ecnoglutide 2.4 mg group. Furthermore, all ecnoglutide doses demonstrated significantly greater reductions in liver enzyme concentrations compared to the placebo group.
Ecnoglutide showed a safety profile similar to that of approved GLP-1 receptor agonists. The most common adverse events were mild-to-moderate gastrointestinal disorders and decreased appetite, which occurred primarily in the dose-escalation period and subsided within a short duration. None of the participants developed pancreatitis, medullary thyroid cancer, or severe gallbladder-related adverse events.
"Ecnoglutide, the world's first biased GLP-1 receptor agonist, marked a milestone in obesity therapeutics with its Phase III SLIMMER data. This achievement represents a significant advancement in weight management," said Dr. Linong Ji, Principal Investigator of the SLIMMER trial. " After 48 weeks of treatment, ecnoglutide achieved a 15.4% weight reduction, with 92.8% of patients attaining clinically meaningful weight loss. Patients in the ecnoglutide 1.8 mg and 2.4 mg groups continued to have weight loss at week 48 without reaching a plateau, indicating that even greater weight loss might be achievable with extended ecnoglutide treatment. Considering the high potency of ecnoglutide, it might also serve as a viable option for individuals who do not achieve sufficient weight reduction with existing GLP-1 receptor agonists at their approved doses or need to achieve a larger reduction in bodyweight.
With China's NMPA review underway and additional clinical trials planned, ecnoglutide is poised to become one of the advanced therapeutic options for obesity and metabolic diseases. Sciwind Biosciences remains committed to advancing this innovative treatment to address the growing global obesity epidemic.
Investor and Media Resources
Full publication: http://doi.org/10.1016/S2213-8587(25)00141-X
ClinicalTrials.gov: NCT05813795
About Ecnoglutide
Ecnoglutide, originally discovered and developed by Sciwind Biosciences, is a first-in-class, long-acting cAMP-biased GLP-1 receptor agonist enhancing biological efficacy and sustainability. Ecnoglutide has successfully completed three Phase III clinical trials, supporting Marketing Authorization Applications in China for treatment of adults with type 2 diabetes, as well as adults with obesity or overweight. From Phase I through Phase III, ecnoglutide has consistently demonstrated a favorable safety profile and strong efficacy, underscoring its potential as a breakthrough therapy in metabolic disease management.
About SLIMMER and the Ecnoglutide Clinical Development Program
The SLIMMER trial represents a pivotal component of Sciwind's comprehensive clinical development program for ecnoglutide, which has totally enrolled over 2,000 participants across multiple clinical trials in metabolic diseases in China and Australia. As the largest Phase III trial of a GLP-1 receptor agonist specifically designed for the Chinese population with obesity, SLIMMER provides robust evidence supporting ecnoglutide's potential as a first-in-class cAMP-biased GLP-1 therapy for weight management.
About Sciwind Biosciences
Sciwind Biosciences is a clinical-stage biopharmaceutical company focusing on discovering and developing innovative therapies to treat metabolic diseases. Sciwind has established a robust pipeline anchored by the lead asset, ecnoglutide (XW003), currently in the NDA stage in China. It has developed multiple proprietary technologies, including oral peptide and long-acting protein therapeutic delivery platforms, and has identified a series of drug candidates based on these core platform technologies. Sciwind has built an extensive pipeline targeting GLP-1 and synergistic pathways, offering both injectable and oral treatment solutions to deliver sustainable and high-quality therapies for patients with metabolic diseases.
For more information, please visit www.sciwindbio.com.
Contacts
General Information: PR@sciwindbio.com
Business Development: BD@sciwindbio.com